RL Blogs

By Process Pro Eric
Jan 08, 2020Breaking down some reaction fundamentals in Refinery Alkylation. |
||||
|
No one can deny the importance of an Alky unit within the integration of a U.S. oil refinery. Whether benefits come from upgrading LPG to gasoline, or offsetting toxics within your gasoline blend pool, the Alkylation process produces liquid gold that all refiners love. This is true whether you operate an HF Alky or a Sulfuric Alky plant.
The Alkylation process is organic chemistry at its finest. In the geekiest form, it is a bimolecular nucleophilic substitution reaction where an olefin molecule reacts with an isobutane molecule to form a branched chain paraffin in the presence of an acid catalyst.
Ok, blah…I did you no favors in demystifying the black box with that last paragraph. However, I did this to prove a point. We can indeed simplify all of this chemistry jargon into something that makes sense. Let’s start from the beginning and break things down into steps.
The alky unit receives olefin feed from upstream cracking units such as the FCC and Coker. Olefins, by definition, are unstable molecules because of the double bond created by an extra set of electrons. This reactivity sets the stage for the alkylation reaction.
Step 1: Cation Formation To start, let’s take a butene olefin in an HF Alky unit. The olefin molecule first reacts with the HF acid catalyst.
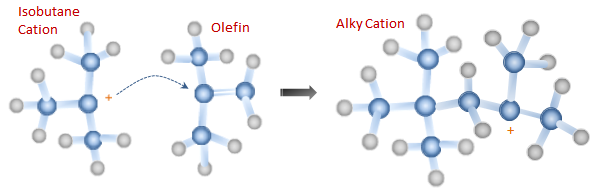
Step 2: Cation Re-arrangement Before combining with an olefin molecule, the butane cation re-arranges itself to form an isobutane cation. This occurs through a hydride and a methyl shift.
Step 3: Pairing The Isobutane cation then reacts with another olefin molecule to create a larger cation
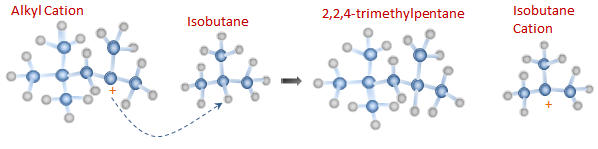
Step 4: Chain Termination To successfully complete the refinery alkylation process, the Alkyl Cation created in Step 3 must now absorb a hydrogen atom from an isobutane molecule.
Step 5: Catalyst recovery Since the role of HF is to act as a catalyst and not as a reactant, the fluoride ions eventually recombine with hydrogen ions to reform a HF molecule again.
Now with the basics of the refinery alky process understood, we can now explain the importance of alky process variables. I’ll address the two most important variables that an alky process engineer monitors religiously.
I/O Ratio: The alky reaction depends on isobutane quite heavily as seen above. Isobutane forms a cation that reacts with an olefin molecule to create a larger chain. Isobutane also provides the hydrogen atom to terminate the alkylation reaction.
Without sufficient Isobutane available, the alkylation reaction can continue to form undesirable longer chain polymers. These polymers not only degrade the value of alkylate in gasoline blending, but can also form acid soluble oils (ASOs) that dilute acid strength.
Acid Strength: Acid acts as a catalyst in a refinery alky reaction, and requires a minimal amount to enable the reaction to occur. As acid strength declines, undesirable side reactions occur, and can cascade on itself in a “runaway” manner that consumes all of the acid in the unit.
An example of an undesirable side reaction is the creation of organic fluorides in an
Under typical circumstances it is normal to see some level of organic fluorides in LPG product (i.e. propane and butane), but a lesser extent in alkylate product. In an acid runaway situation, the amount of organic fluorides in the alkylate product elevates as acid is consumed and exits with the alkylate.
By reviewing the fundamentals of the refinery alkylation reaction, you can now apply this knowledge in understanding how the different refinery alky products are formed. Use of the 5 Steps above can explain what happens when you alkylate different types of olefin feedstocks.
At the basic level you can see how different products are obtained when alkylating Propylenes, Butenes, and Amylenes. It’s intuitive to derive the production of C7, C8, and C9 alkylates.
It’s even more fascinating to understand how different isomers of alkylate are produced from the different isomers of olefin feed. That’s right…1-butene, cis 2-butene, trans 2-butene, and isobutene all produce different types of alkylates. From 2,2,3-trimethylpentane to 2,4 dimethylhexane, there’s another sub-level of chemistry geekiness that I’ll save for a later discussion.
For now, I trust that the basic chemistry lesson above is enough to demystify the black box a bit. | ||||
|
Related Blogs |
||||
|



.png)
.png)