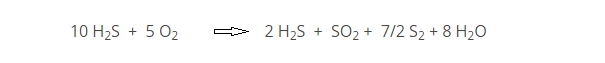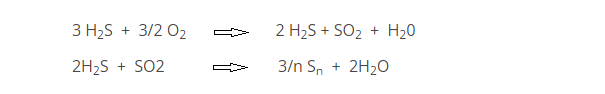RL Blogs

By Reactor Resources
Sep 17, 2018Overview of how refineries complete the sulfur recovery process |
||
stoichiometric oxidative environment and the second section is catalytic reaction converting gaseous sulfur species to elemental sulfur.
Process and Reaction Mechanics
Utilizing the chemical reactions of the Claus process, the multi-stage oxidation of H2S is guided by the overall reaction:
The first reaction step is the thermal treatment of the refinery acid gas. Air (or oxygen enriched air) is added such that only one-third of the H2S is oxidized to SO2. The remaining two-thirds of oxygen reacts with the products produced to complete the conversion to elemental sulfur. A catalytic step follows where H2S and SO2 react at lower temperatures over alumina. The equations below highlight the primary chemistry occurring in the SRU.
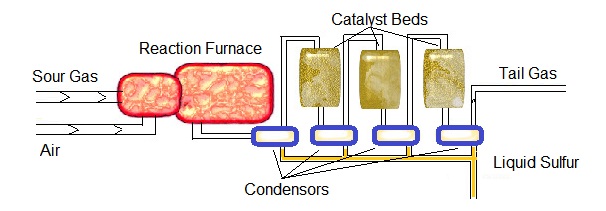
Generic Diagram of SRU
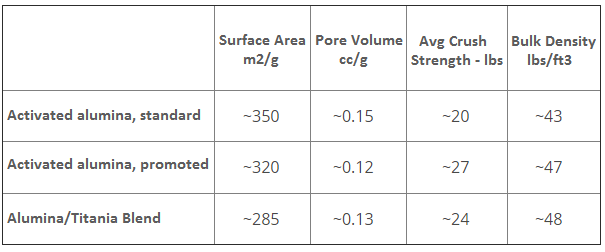
Effective catalysts for the Claus reaction generally utilize activated alumina. This material is an extremely porous aluminum oxide. The qualities that are critical to optimum catalytic performance are developed during the thermal activation of the aulmina:
Non-promoted aluminas are the most popular materials used for Claus processing. However, some applications may require promoted activated alumina, titania/alumina, or even titanium dioxide catalysts.
Alumina materials are susceptible to deactivation through a number of mechanisms, both physically and chemically. Aging occurs due to a degradation of the physical structure of the alumina. Pore volume can change. And pores can become blocked as well, reducing active surface area and retarding conversion activity. However, sulfate poisoning or sulfation is the primary culprit that causes performance decline. Carbon deposition can also play a role, also obstructing access to the active surface.
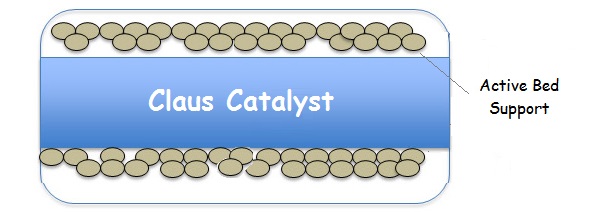
Claus Catalyst General Specifications
A promoted Claus catalyst typically will contain a promoter of 1-5% Na2O (sodium oxide). The promoter retards the conversion of the sulfide species to sulfur. The unabated reaction can cause sulfation (sulfur layering) on the surface of the catalyst. Promoted alumina has also shown improved COS (carbonyl sulfide) decomposition over time.
A typical sulfur recovery unit Claus bed is diagramed below.
Claus units are designed to convert H2S and SO2 to elemental sulfur. However, the catalyst must also have good activity for conversion of CS2 (carbon disulfide) and COS (carbonyl sulfide). CS2 is a by-product that forms in the reaction furnace from impurities in the acid gas. COS is formed by the presence of CO2 and CO. CS2 is harder to convert, as high temperatures are required for the reaction rates.
Claus conversion occurs very quickly in all converter beds, but is incomplete because of equilibrium limitations. Low temperatures in adsorber beds favor higher conversion. Lower inlet temperatures are used in successive adsorber reactors to maximize sulfur make.
An optimized Claus plant with three catalytic stages can achieve sulfur recovery of 96-98.5%.
Catalyst Deactivation
The alumina in the Claus process deactivate over time. There are four main mechanisms for deactivation: hydrothermal aging, sulfation, carbon deposition and liquid sulfur deposition.
Steaming or heat and water vapor cause surface area degradation. This is a slow process that will eventually render the alumina unreactive for sulfur conversion.
The most destructive mechanism is sulfation. The formation of sulfur oxyanions on the surface of the alumina blocks active sites. Sulfation is determined by several factors. Lower temperatures drive sulfation, which means that sulfation is more favorable in the third bed and moves forward. Sulfur build up is also more prevalent in the presence of oxygen.
Catalyst is also deactivated by coke deposition. Heavy hydrocarbons from feed can attach to the surface of the alumina, thereby blocking active sites. Lastly, liquid sulfur can form on the surface under low reactor temperatures, resulting in the pores being filled up. Raising the temperature reverses sulfur blockage.
Catalyst deactivation generally involves surface area and active site obstruction, so a catalyst with a better pore structure will have a superior performance.
Claus Market
Claus catalyst usage is determined by total SRU capacity at a facility. The United States has approximately 130 petroleum refineries, of which more than 110 have at least one SRU on-site.
The amount of alumina loaded in an SRU is dependent on the size of the SRU. A typical SRU is measured by the tons of sulfur make per day. An average sized SRU is 100 tons a day of sulfur make. There is more than 65 million tons of sulfur produced worldwide from hydrocarbon facilities. | ||
|
|