RL Blogs

By Junaid Shah
Sep 26, 2016Introductory basics of refinery naphtha hydroprocessing. |
| Naphtha hydroprocessing is required in a refinery to accomplish various objectives. These activities can range from preparing feed for downstream processing to removing impurities from gasoline blending components.
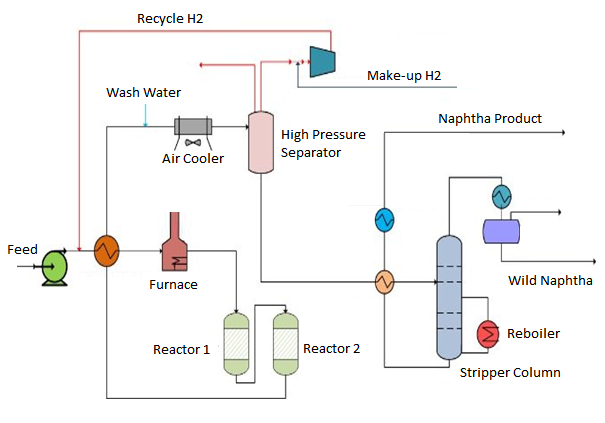
A schematic of naphtha hydrotreating unit is presented below:
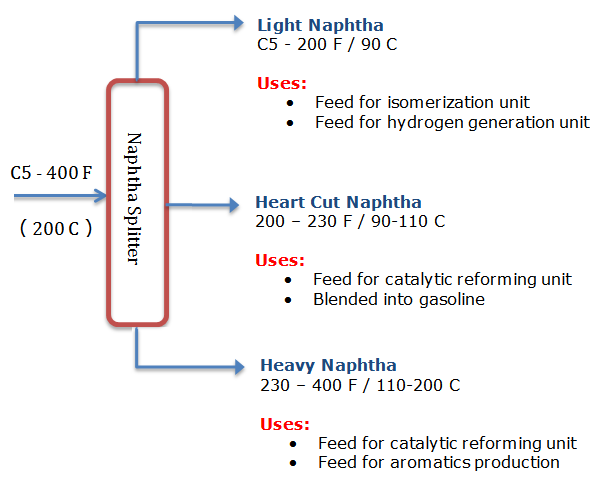
The Naphtha cut, which is obtained from crude, is typically defined as C5s through 400 deg F (200 deg C) boiling range material. In refinery lingo this is referred to as full range naphtha, but it can be broken down into further sub-cuts as defined per below.
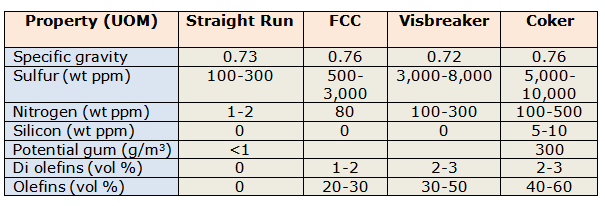
The naphtha that is available to a refinery is derived from various units within the refinery itself. We can have straight run naphtha (SRN i.e. directly from Crude distillation unit), FCC, Visbreaker and Coker naphtha. The typical properties of these different types of naphtha are summarized as below.
As is clearly evident, when we move from SRN to Coker naphtha the quality of naphtha degrades with a monumental increase in impurities such as sulfur, nitrogen, silicon etc. The summary above implies that coker naphtha processing requires more severe hydroprocessing conditions than any of the other naphtha streams.
The extent to which impurities need to be removed from naphtha will be governed by the end use of naphtha. For example, feeding naphtha to a catalytic reforming unit requires a sulfur content below 0.50 ppm in order to safeguard the catalyst against poisoning, while sending naphtha directly to a gasoline blend pool may only require a sulfur content of 30 ppm.
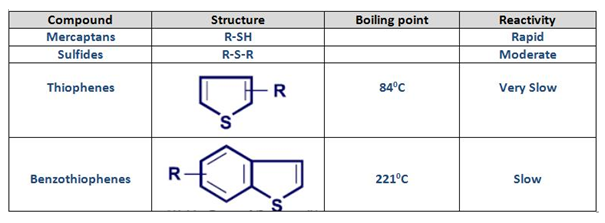
Sulfur bearing compounds in species in naphtha:
The typical classes of S bearing compounds present in naphtha are illustrated in the table as below. In refinery hydrotreating, thiophenes and benzothiophenes are the least reactive molecules, especially benzothiophenes in which removal of sulfur is stearically hindered.
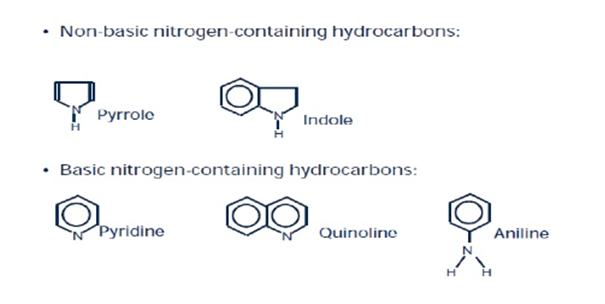
Nitrogen bearing compounds in naphtha:
Nitrogen containing compounds in naphtha broadly fall into two classes. Non basic nitrogen containing hydrocarbons such as pyrole, indole etc. and basic nitrogen containing hydrocarbons like pyridine, quinoline, aniline, etc. Basic nitrogen containing species are detrimental for the catalyst as they reduce the acidic function of the catalyst.
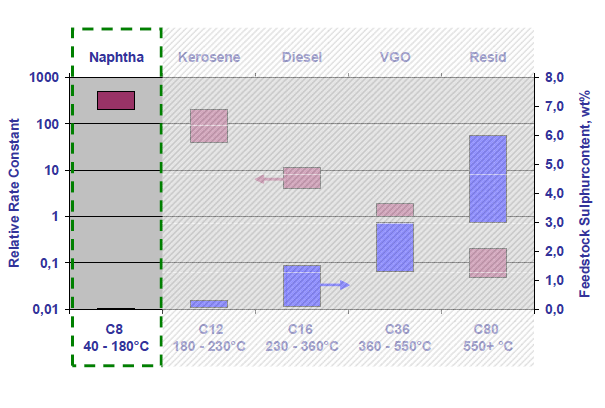
Reaction rate constants and kinetics:
For main hydroprocessing reactions carried out on naphtha (i.e. desulphurization and denitrification), the relative rate constants are higher than the subsequent hydrocarbon cut which happens to be kerosene (i.e. Jet fuel). Hydroprocessing of naphtha involves heterogeneous catalysis which means that naphtha in vapor phase is bought into contact which a solid catalyst.
The reaction between reactants and catalysts is limited by diffusion and follows first order kinetics. Hydrodenitrification (HDN) is much slower than hydrodesulphurization (HDS) reaction. Apart from HDS and HDN, other reactions that are encountered in naphtha hydroprocessing are olefin saturation, hydrodearomatization (HDA), halide removal, hydrodemetallization and oxygenate removal.
The kinetic rate of reaction for HDS in naphtha cut is presented in the figure below.
Now based upon the kinetics and contaminants present comes the most important question.
What is the choice of catalyst?
Should I go with a NiMo (Nickel Molybdenum) catalyst or will CoMo (Cobalt Molybdenum) catalyst work better for me?
It’s the presence of nitrogen that will determine the catalyst selection. In simple terms, CoMo catalyst performs well for feeds bearing higher nitrogen in comparison to NiMo catalyst. That is because basic nitrogen carrying molecular species are less inhibiting for CoMo catalyst as compared to a NiMo catalyst.
Also to note for HDS reactions, CoMo catalyst has lower hydrogen consumption in comparison to a NiMo catalyst.
CoMo catalyst achieves direct HDS in a single hydrogen consuming step whereas NiMo achieves HDS in two steps, which both involve consumption of hydrogen. For this reason, the refinery hydrogen balance should also be a consideration for catalyst selection. Refineries constrained by hydrogen supply should consider higher loading of CoMo catalyst.
Now that we have chosen a catalyst, let's determine the operating temperature and pressure. The maximum operating temperature for a hydroprocessing reactor is often set by the recombination temperature of H2S (i.e. mercaptan recombination), which happens to be a reaction product of the HDS process.
The reaction mechanism in HDS involves formation of a carbocation. At the reactor outlet H2S again reacts with unstable reaction intermediates forming R-SH. Generally operation at 600 – 645 F (315 - 340 C) average reactor temperature will give acceptable rates of desired reactions and will not result in significant H2S recombination.
Now coming to the pressure part of the process. Adequate hydrogen (H2) partial pressure throughout the catalyst bed is a very important process parameter. H2 is partly consumed in hydroprocessing reactions and presence of adequate H2 at active sites of catalyst limits deactivation by inhibiting coke formation.
At higher pressures, reaction can nearly achieve completion and the catalyst remains active for a longer time. For SRN HDS reactions 500 psig (35kg/cm2) reactor pressure is generally employed, while cracked naphtha feedstocks containing higher levels of nitrogen and sulfur bearing impurities require higher processing pressures, up to 800 psig (55kg/cm2).
Most naphtha hydroprocessing units are designed so that at their operating pressure, HDS and HDN reactions are substantially completed well below the design temperature of the reactors for a particular feedstock. Since the reactions are limited by diffusion, the catalyst bed particles must be small where feasible. The chosen catalyst must also have large pore sizes.
For units processing cracked feedstock a graded bed is recommended. The bed grading also serves an important purpose of limiting the pressure drop within the reactor. Grading takes care of any scale, fines, metals that may ingress into the reactor along with the feed. Active grading material can also be employed for silicon (Si) deposition, diolefin and olefin removal. Diolefins lead to formation of gums/polymers within the reactor, which increases pressure drop for the catalyst bed.
At core of your hydroprocessing success lies a well-managed process with superior control and analytics.
Your valuable feedback, suggestions and rich experiences to further improve this post are eagerly awaited. |
|
|