RL Blogs

By Process Pro Eric
Jul 08, 2012Distillation is a core refinery process that remains fundamental to economic operation. Successful job performance for process engineers and operators depends on quality distillation monitoring and troubleshooting. |
||||||
Distillation Fundamentals
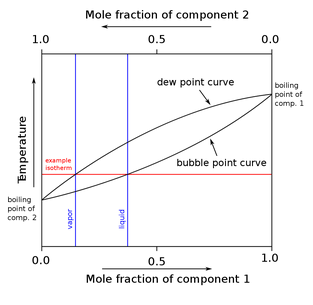
Distillation is simply the separation of molecules (or components) based on their relative volatility. Another way of thinking about it, distillation separates components based on their relative boiling points. Since chemicals or components such as gasoline, jet, and diesel boil at different temperatures distillation columns can separate mixed feed streams into distinct product streams.
pressure. Since oil distillation isn’t as simple as two-component separation, it’s necessary to use equilibrium ratio or K factor to understand the relative volatility of separations. The K factor is the ratio of vapor moles and liquid moles in a separation stage. When thinking about distillation basics it’s important to remember the basic fundamentals of the McCabe-Thiele method and Raoult’s Law.
mass balance monitoring pro. This is as simple as calculating the mass into the column versus the mass out of the column. Mass balances can help engineers and operators verify that unit telemetry devices (orifice plates, flow meters, and level controls) are operating properly.
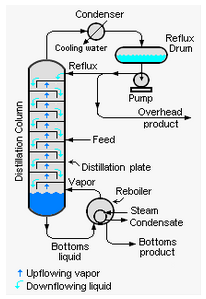
The second distillation basic is that a distillation column must be energy balanced. Nearly every distillation column has a reboiler and a condenser that adds and removes energy from the distillation column. This driving force creates the temperature gradient in the equilibrium flash stages to separate light and heavy molecules.
The principle of evergy balance is critically important for separation efficiency and quality. For instance, if a distillation column pump-around takes out to much energy, then the distillation will result in poor separation quality or no separation at all. Conversely, too much heat input to the column can be just as detrimental to separation efficiency.
Finally, an important distillation principle that is often over looked by operators and engineers is that the distillation column must pressure balance. Put another way, distillation column pressure gradients are important for understanding separation quality and efficiency. Just like temperate, the gradient of pressure up and down the column is what allows for the gradient of compositions in the equilibrium flash stages.
Of all the above basic, pressure is often the easiest to monitor and diagnose because it takes the least amount of computational analysis. An engineer or operator can plug in a pressure gauge almost anywhere in a distillation column to understand the pressure gradient between stages (trays).
Distillation Troubleshooting Tips and Tools
Don’t trust the instruments. Whenever possible, use basic tools to understand the temperature, pressure, and flow in the distillation column. Half the time the root problem in a distillation column is a faulty temperature, pressure, or flow reading.
Mass balance the column. Engineers should have routine monitoring in place to understand the column mass balance. This can be used to diagnose faulty flow meters, level controllers, or lab/analyzers results. Reliance on good flow and level instruments is important for further troubleshooting efforts.
Understand the column heat balance. While this analysis is easy to conceptually understand, the practical application can be rather difficult. There are several layers to column heat balance. A simple overall heat balance can be an invaluable tool. At minimum, a distillation column should have a good heat balance around the condenser, reboiler, rectifying section, and stripping section. The ambitious engineer would also benefit from a tray by tray heat balance.
Temperate gradient survey. A “bootleg” energy balance can be achieved by monitoring the temperature gradient throughout the distillation column. This can be achieved through internal or external shell temperature couples. The most important aspect is to ensure that there is a temperature gradient down the distillation column. This ensures the that there is a separation gradient down the column as well.
Distillation temperature gradient. Another simple monitoring method around the heat balance is to monitor the distillation temperature difference between products. By simply measuring the 95% point of the light component versus the 5% point of the heavy component an engineer can understand if the separation quality is changing over time. A deviation of “normal” can be a sign that something is wrong with the distillation process.
Understand the pressure balance. Routine monitoring of the pressure gradient between stages is critical to understanding the performance of a distillation column. It’s important to remember to measure pressure drop of the liquid in the column so don’t forget to calibrate the pressure readings to the specific gravity of the fluid in the column.
Higher than normal pressure drop can indicate flooding. Lower than normal pressure drop can indicate weeping. Both flooding and weeping significantly reduces the performance of a distillation column. Another good idea is to understand the pressure drop as a ratio of the distillation column tray spacing. Anything greater than 25% probably means flooding.
Understand Reflux Ratio. Every distillation column has a critical vapor and liquid ratio in the rectifying section of the distillation column. Engineers and operators should understand the affects of varying reflux ratio on the distillation quality. Understanding “normal” can help diagnose problems when they arise. (But don’t forget the mass balance relationship)
Understand Stripping Ratio. Similar to the reflux ratio importance, understanding the vapor and liquid ratios in the stripping section as function of heat input to the column can help diagnose distillation problems as they arise. (But don’t forget the mass balance relationship)
X-Ray the distillation column. Depending on the size of the distillation column, X-Raying the distillation column can help an engineer understand the physical structure inside a column. This can be very helpful when suspecting a mechanical defect or to aid with the other troubleshooting tips above.
Gamma Scan. Gamma Scanning is performed using a small radioactive source and a sensitive electronic detector. The source and detector are kept external to the tower and positioned on opposite sides at a fixed distance apart. Gamma rays travel from the source through the tower to the detector where they are counted.
A density profile is generated when gamma rays are moderated by the contents inside the vessel. Vapor allows more gamma rays to pass through and therefore appears less dense than a liquid or solid. This can be used to confirm the flooding, weeping, or physical damage inside a distillation column.
Distillation is probably the most important process in a refinery. Therefore, adequate control and monitoring are critical to the success of the refinery. Distillation monitoring and troubleshooting help keep energy cost down and profits up. The importance of simple distillation can be illustrated by the economic penalty of burning propane in refinery process gas. | ||||||
|
|